The Ethics and Public Policy Center (EPPC) released a study of over 865,000 women who took the abortion pill. Their data reveal that the risk of suffering serious adverse events was over 10%–in sharp contrast to the FDA’s claim of 0.5%, (which was based on only 30,000 women and decades old data).
The EPPC used real-life data from insurance claims during 2017-2023 that included 865,727 prescribed mifepristone abortions. The authors looked at the incidence of adverse events occurring up to 45 days after taking the abortion drugs. What they found is that 10.93% of women who took the abortion pill protocol suffered a serious adverse event. Another way of expressing this is that the actual risk of suffering an adverse event after taking the abortion pills is 22 times higher than the FDA’s claim (21.86 x 0.5 = 10.93).
Following the FDA’s criteria for adverse events (AEs), the study only included AEs categorized as “serious” and “life-threatening.” Mifepristone labeling lists serious adverse events from clinical trials as sepsis, infection, transfusion, hemorrhage, hospitalization, and emergency room visits. The study focused on abortion-related hospitalizations and emergency room visits identified by diagnosis and procedure codes. In addition, they included other events deemed serious or life-threatening.
This included:
- Sepsis, infection
- Hemorrhage, transfusion
- ER visits, hospitalizations
- ectopic pregnancy
- Repeat abortion (surgical)
- Blood clots
- Anaphylaxis
- Other life-threatening events (cardiac, pulmonary)
The study purchased a comprehensive U.S. health insurance claims database (2017-2023) with de-identified data (visits, diagnoses, procedures, prescriptions from private, Medicaid, Medicare, TRICARE, and VA). This type of database is commonly used in academic and regulatory research.
Note: if a woman experienced more than one adverse event, she was only counted once.
The chart below summarizes how the FDA has progressively removed safety measures put in place to make the abortion pill protocol less risky. (The protocol can never be “safe” since it ends the life of a human being and causes harm to the mother and family.)
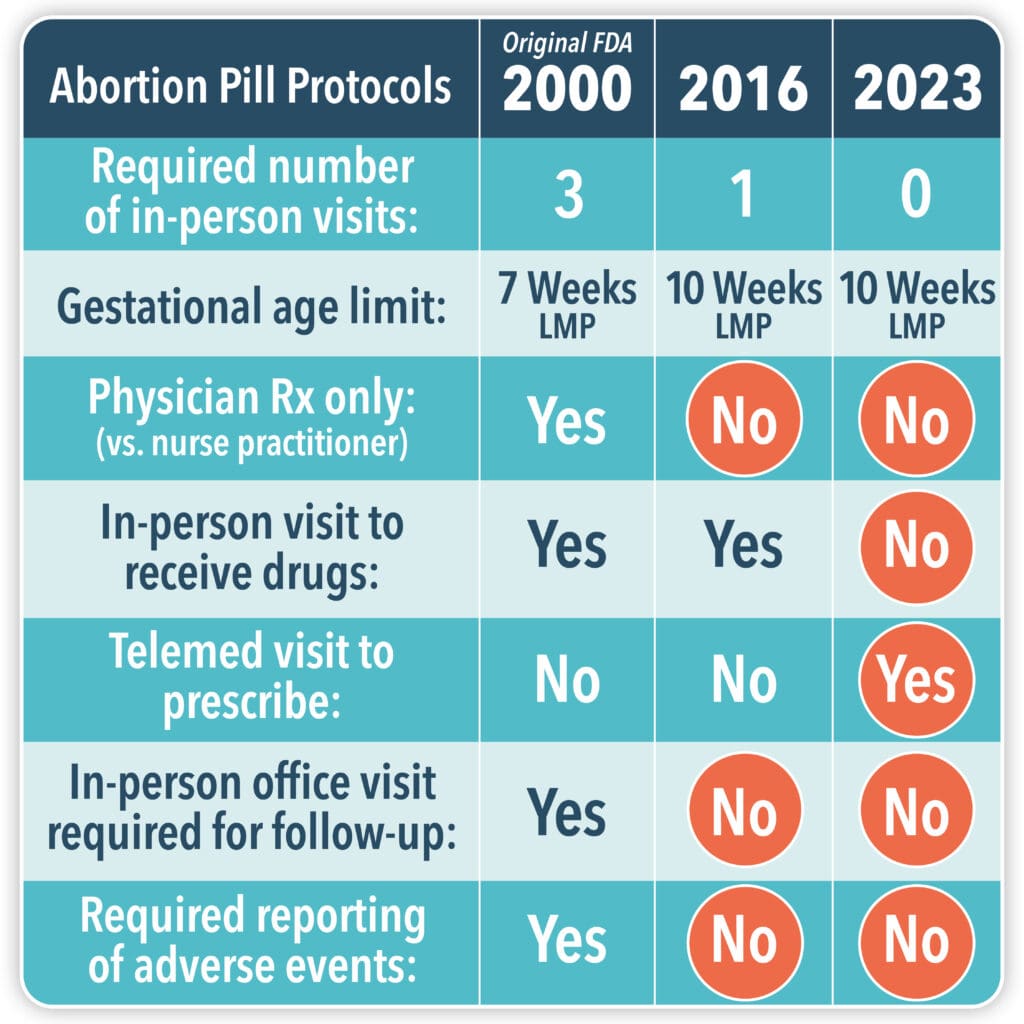
Note that in 2016, the FDA stopped collecting adverse event reports altogether! They only wanted to know if the mother died. That makes no sense when you consider that the abortion pill protocol is one of approximately 77 FDA approved medications that require a REMS due to the increased risk of adverse events!?
It is clear that removing common sense safety measures has dramatically increased the complications that women are experiencing. Remember that the FDA’s initial approval was based on data derived from women who had taken the abortion pill protocol after confirming their pregnancies, and seeing a doctor in person on three separate visits. The EPPC’s study looked at women’s experiences with taking the abortion pill protocol from 2017 to 2023 with little to no direct physician oversight, and in many cases, no confirmation of pregnancy.
Opponents dismiss this study because it was not published in a peer reviewed journal. There was a time when peer-reviewed journals were the gold standard for providing trustworthy, unbiased scientific reports. Sadly, that is no longer the case. It is common for authors who study abortion risks and other controversial subjects, to encounter obstacles to having their work published due to bias and political agendas on the part of the editors. We still need scientific peer-reviewed journals, but other data sources are valid, too.
Regardless of one’s view on the scientific process, the data revealed in the EPPC study should be an eye-opener for anyone who cares about the real-world experience of women taking these abortive drugs.
Medical professionals attest that if they were routinely performing a procedure on patients that had a 10% risk of serious adverse events they would be out of business. When one considers the number of abortions done in the U.S. alone on a yearly basis, a 10% risk is a staggeringly large number of affected women!
The authors are calling on the FDA to:
- At a minimum: Reinstate the original stronger safety protocols (3 visits, must see a physician, no telehealth care, use up to 7 weeks LMP)
- Mandate adverse event reporting (not just maternal death)
- Investigate current reports and consider removing the abortion pill protocol from the market
Take Action
The pro-life community is encouraged to reach out to legislators, the FDA, and the Federal government to respond and take a stand to protect women.
Care Net joined over 100 pro-life signatories in a letter to the HHS urging the FDA to prioritize women’s safety by reinstating prior safeguards for mifepristone prescriptions and thoroughly reviewing data showing the drug’s lack of safety and efficacy. The letter highlighted the study’s findings and asserted the FDA’s crucial role in ensuring the safety, efficacy, and security of medical products. It argued that the FDA’s credibility and American women’s health depend on addressing the documented harms associated with mifepristone.
You can take action now by clicking on Stop Harming Women.